For Precious Lives and Smiles

Promote Development to Deliver Better Medical Devices to Patients.
HI-LEX will continue to contribute to medical activities that save lives with new technologies.

Philosophy
1. Contribute to Society
2. Commit to Fostering Talents
3. Bring Trustworthy and Innovative Medical Devices to Society
Our Products
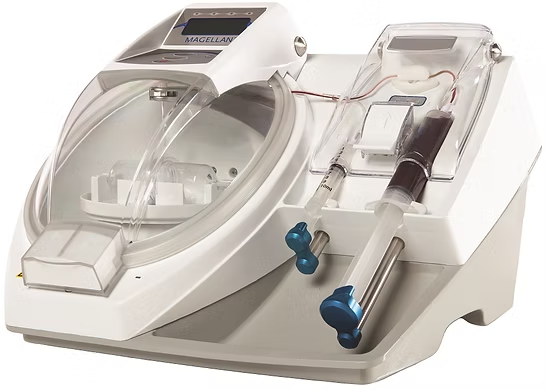
MAGELLAN®
Autologous Concentration System(Class III)
Approval No.: 23100BZX00024000
MAH: HI-LEX medical inc.

EVAHEART®2
Left Ventricular Assist Device (LVAD, Class IV)
Approval No.: 22200BZX00939000
MAH: Sun Medical Technology Research Corp.

HI-LEX GRAFT®PLUS
ePTFE Vascular Graft (Class III)
Approval No.: 20200BZZ00079000
MAH: HI-LEX Corporation
Company Profile
| Name | HI-LEX medical Inc. |
| CEO | Taro Teraura |
| COO | Masuyuki Hada |
| Executive Officer | Yasuo Seki |
| Auditor | Koichi Matsumoto |
| Incorporated | August 30, 2021 |
| Location |
4-24-3 Koto-Bashi, Sumida-ku, Tokyo, Japan
|
| Capital Stock | ¥100 million |
| Licenses and Certifications |
First Class Medical Devices Marketing License (License Number: 13B1X10370) Marketing and Leasing License for Highly Controlled Medical Devices (License Number: 3-sumi-huku-eisei-yaku-569) Repairing Medical Devices (License Number: 13BS201596) Secondhand Dealer (License Number: 307732420934) |
| Type of Business |
Import, sales, repair and maintenance of medical devices Support activity related healthcare Support services for medical device development |
| Related Companies |
HI-LEX Corporation and
its Medical Device Dept.
Sun Medical Technology Research Corp. EVAHEART, Inc. |
